Simple 3-Step Process
From document upload to validated E2B(R3) in minutes
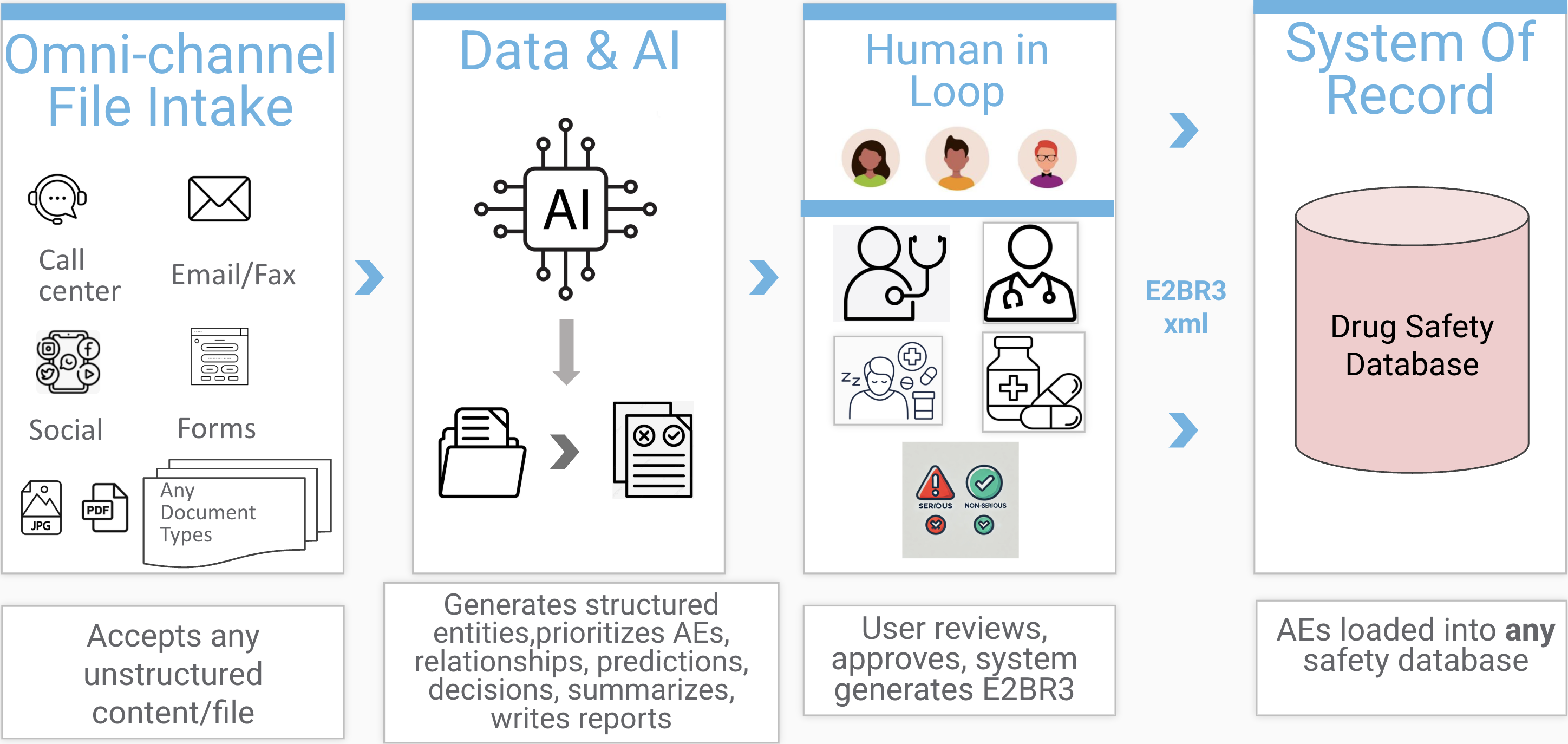
Process any document in any language. Generate ICH E2B(R3) XML in minutes, not hours. First AI-generated ICSR submitted to EMA.
Built for pharmaceutical companies, validated for regulatory compliance
Process PDFs, emails, faxes, handwritten notes. Our AI handles 100+ languages with medical terminology.
Generate ICH-compliant XML with built-in business rules validation. Export to Argus, Veeva, ARISg.
Complete audit trails, e-signatures, role-based access. SOC 2 Type II and ISO 27001 certified.
Review and edit before submission. Track changes, add comments, maintain full control.
Automatic coding to latest MedDRA version. WHO-Drug dictionary integration available.
GDPR, HIPAA compliant. Data residency in EU or US. Your data never trains our models.
From document upload to validated E2B(R3) in minutes

"A Milestone in Pharmacovigilance: Wörwag Pharma submits its first AI-generated ICSR to the EMA"
Join leading pharmaceutical companies using TheraLyze.ai
🚀 Signup Now